
Plastic pollution is a global problem that continues to grow. Over 400 million tons of plastic is produced every year, and each year manufacturers make more than the year before. Despite the high energy and carbon value in plastics, and the large investment to initially create these versatile materials, most plastic is never recycled. In fact, less than 10% of plastic has ever been recycled. Instead, it is piling up in landfills, collecting on the sides of the roads and along beaches, ultimately polluting our lands and waters.
An interdisciplinary team of scientists, led by researchers at the U.S. Department of Energy’s (DOE’s) Ames and Argonne National Laboratories, have banded together to develop new ways to chemically recycle plastics and transform them into more valuable products, a process called upcycling.
“At iCOUP, we are developing the science needed to advance technologies that can address the plastic waste accumulation problem.” — Aaron Sadow, director of the Institute for the Cooperative Upcycling of Plastics (iCOUP)
This effort is led by the Institute for the Cooperative Upcycling of Plastics (iCOUP), a DOE Office of Science Energy Frontier Research Center. iCOUP brings together chemists and materials scientists from the national labs and universities across the country. Their goal is to develop the chemical pathways that will enable companies to turn plastic waste into valuable commodities.
The truth is, plastic is hard to recycle. Plastic is made of long chains of molecules called polymers. These polymer chains make plastics strong and durable, but once the chains are broken, they are very difficult to put back together again, and the plastics’ properties degrade. Melting and remolding during recycling tend to break some chains, producing plastic typically of a lower quality than the original material. In addition, most plastics are made from inexpensive petrochemicals, and the cost to manufacture new plastics is less than the cost to recycle. Plastics are also designed to be durable, so it can take more than 1,000 years for a piece of plastic to break down in a landfill.
But what if plastics never made it into the landfill in the first place?
“At iCOUP, we are developing the science needed to advance technologies that can address the plastic waste accumulation problem,” said Aaron Sadow, the director of iCOUP and a chemist at Ames Lab. “If successful, we can develop a carbon cycle that takes the carbon in plastics and recycles it in the form of other chemicals and materials.” Ultimately, this could make plastics part of a circular economy, where plastic is never sent to a landfill.
The iCOUP team is developing new chemical recycling methods to make sustainable, high-quality plastic materials and transform plastic into valuable chemical products, such as lubricants and surfactants, which reduce the surface tension of a liquid.
“In 20 or 30 years, we want to use the technology we develop at iCOUP to make new products from plastic waste that is currently lost in the environment,” said Massimiliano Delferro, a chemist at Argonne and the deputy director of iCOUP. “But scientifically, that’s a big challenge.”
Their bold vision recently led to a string of successes.
The iCOUP team is developing ways to take the large molecules that make up plastic and selectively convert them into saleable chemicals. Over the past few months, the iCOUP team published several scientific papers on their various approaches to this problem.
- Making plastics easier to recycle: The iCOUP team developed a way to break up plastics and transform them into a new polymer that is biodegradable and easier to recycle. The new chemical recycling method created plastic products that were of similar quality to the plastic waste. Not only that, but this process allowed for the plastics to be recycled repeatedly without degrading the quality of the plastic.
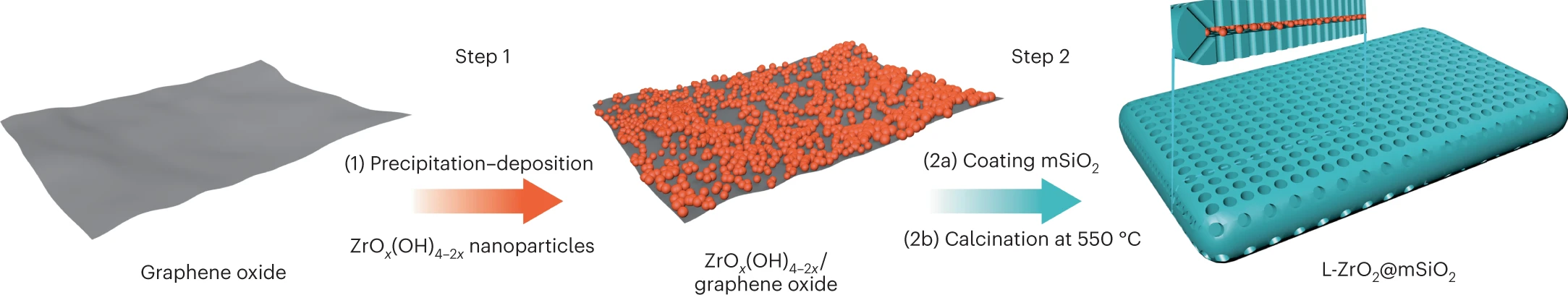
- Designing a new catalyst: The iCOUP team developed a new zirconia-based catalyst to separate polymers in plastic waste. Current catalysts are based on expensive rare earth metals, such as platinum. Zirconia is an effective alternative that is relatively inexpensive and abundant. By suspending ultrasmall zirconia nanoparticles in silica, the scientists were able to separate the long plastic polymers at specific intervals, creating new high-value chemicals at low cost.
- Developing an efficient way to make a catalyst: The iCOUP team developed a more efficient way to make a certain catalyst that transforms plastic waste into a lubricant. This catalyst was originally created by their partners at Northwestern University. The catalyst is very effective, but difficult to produce. The iCOUP team found that an easier and less expensive chemical technique could be used to produce large amounts of the catalyst so it could eventually be used in commercial applications.
- Creating valuable products from plastic waste: Most recently, the team found a way to add functionality to the plastic polymers once they are broken apart. This process involved chemically modifying the plastic waste so it can be used in more valuable products, such as surfactants or emulsifiers, which help keep two liquids mixed.
This research was possible because of the tools and expertise only available at the national labs, including the Advanced Photon Source (APS), a DOE Office of Science user facility at Argonne.
“In this latest round of research, the APS successfully delivered molecular-level information about the newly developed catalysts, elucidating how they function and providing a technical clue toward higher performance,” explained Byeongdu Lee, a physicist at Argonne and a group leader at the APS. “This work shows how the knowledge collected from this powerful X-ray facility can be put to use to solve some of the world’s biggest problems, such as plastic pollution.”
Researchers relied on another DOE Office of Science user facility, the Center for Nanoscale Materials, to measure the size of the new polymer particles they were creating. And for one project they used Argonne’s Materials Engineering Research Facility to help scale up the production of their new catalysts.
At Ames Lab, the team took advantage of the electron microscopy available at the Sensitive Instrument Facility, where Frédéric Perras characterized the new catalytic materials. They also used the Solid State Nuclear Magnetic Resonance Laboratory to help them understand the interactions between polymers and the new catalysts.
“It’s a wonderful collaboration that makes use of the expertise and facilities available at both labs,” Sadow said. Delferro added that “the heart of iCOUP is really the collaborative environment. We take full advantage of all the DOE facilities that we have at the center to really try to answer big scientific questions within the plastic upcycling problem.”
As the iCOUP team continues to develop new ways to upcycle plastics, they have their eye on the next step: getting the technology to market. They have already started working with companies, including Chevron Philips and a startup launched by their former iCOUP post-docs, to commercialize their technologies.
“Our goal now is to create a product that companies can use in a way to make a profit,” said Magali Ferrandon, a chemist at Argonne and part of the iCOUP team. “We want to make it cheaper and easier for companies to be able to do this commercially.”


